The Discovery of a New Class of Compounds
Research
What is Inflammation?
Inflammation is the body’s first-line defense after injury or infection.
For more than 2000 years we thought that inflammation resolved when the stimulus went away.
Currently, we use anti-inflammatory drugs to inhibit inflammation. But most of these drugs depress resistance to infection and have multiple side effects, delay the resolution of inflammation and prolong the infection.
Inflammation brings healing mechanisms to the site of the problem.
Inhibiting these chemical mediators to reduce the signs of inflammation does not solve the problem!
Previous Thinking
The five signs of inflammation have been known for more than 2000 years. Roman physician Aulus Cornelius Celsus described them as:
- Calor (heat)
- Rubor (redness)
- Tumor (swelling)
- Dolor (pain)
- Functio laesa (loss of function)

Rudolph Virchow
We are using drugs to stop acute inflammation,
but these drugs also block the body from being fully engaged in resolving inflammation and result in multiple side effects.
Discovery
Resolving & Regenerating
The body has the potential to achieve full regeneration, if excess inflammation does not get in the way.
NOCENDRA’S leading scientists are at the forefront of this discovery and are developing new drugs to enhance the body’s natural healing capabilities by limiting excess inflammation.
New research shows that the body has the capability to produce molecules that resolve inflammation naturally while enhancing the immune system. They have dual actions controlling inflammation and promoting healing with no known side-effects.
Our scientists have realized that the body can heal itself completely, without scarring, if we can properly regulate inflammation.
Natural Control Mechanisms: Specialized Pro-resolving lipid Mediators (SPMs)
The body has natural dual actions mediators.
SPMs both initiate and regulate inflammation, therefore, SPMs are both anti-inflammatory and pro-resolving.
The role of SPMs in inflammation
Reverse the five cardinal signs of inflammation
Induce secretion of healing molecules
Remove dead cells
Clean-up debris
Stimulate the release of other SPMs changing the inflammatory status of the whole body
SPMs are agonists; they bind to a cell to change the cell’s activity from pro-inflammatory to pro-resolution.

Pre-Clinical Studies
Rabbit Periodontitis
Pre-clinical studies were done in rabbits; periodontitis was induced with a human pathogen.
Measures were made of tissue changes – pocket depth and bone changes – infrabony pockets.
The 12-weeks experiment was able to demonstrate that our product showed:
No toxicity
Abatement of the disease
Regeneration of the tissues
Conclusions from Pre-clinical Studies
The Role of SPMs in Periodontal Regeneration:
SPMs reverse periodontal inflammation
SPMs arrest progressive periodontitis
Elimination of inflammation promotes spontaneous regeneration of lost tissues
Outcomes
Reduction in Soft Tissue-Pocket Depth Measurements

Hard Tissue-Infrabony Pocket Depth Measurements
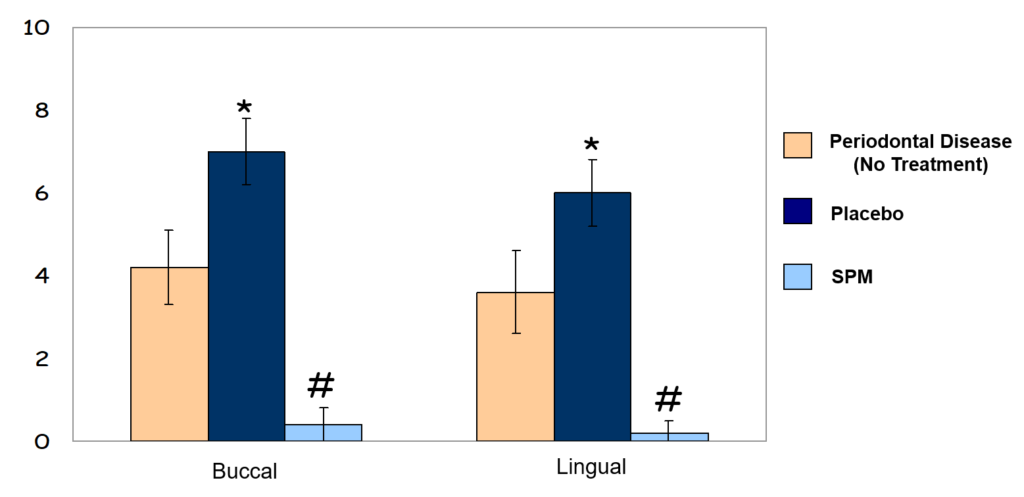
* p <0.05 compared to “Periodontal Disease Group”
* p <0.01 compared to both “Periodontal Disease Group” and “Placebo”
Phase I
Human Periodontitis
Phase I Clinical Trial is completed and has assessed the Safety and Preliminary Efficacy of Lipoxin Analog SPM Oral Rinse for the treatment of Gingivitis.
125 subjects with Gingival inflammation were part of the trial which lasted 28 days.The results have shown that SPM is safe and reduces Gingivitis
We are now ready to go into Phase II to test the optimal dose and determine the impact of the drug in periodontal disease.
Statistical Analysis
- Descriptive statistics (means, standard deviations, and ranges) were calculated for all outcomes separately by treatment group (SPM rinse, placebo rinse and no-rinse control)
- Linear regression with robust standard errors were used to estimate treatment group differences at each time point and treatment group differences in change from baseline for each time point and corresponding 95% confidence intervals (95% CI).
- All analyses were done on a site-specific basis and the estimates represent differences in mean site-specific changes between treatment groups.
- Post-hoc differences and p-values were estimated for treatment group differences using ANOVA and Fisher’s least significant difference (LSD) method.
Conclusions from Phase I
Primary outcome Measure: Safety
Treatment with SPM was safe and well tolerated in this study.
Phase II Trial is expected to start in the 2nd Quarter of 2020 to determine the optimal dose. Dosage analysis will also provide significant efficacy data.
Change in Gingival Index (Mean + SEM)
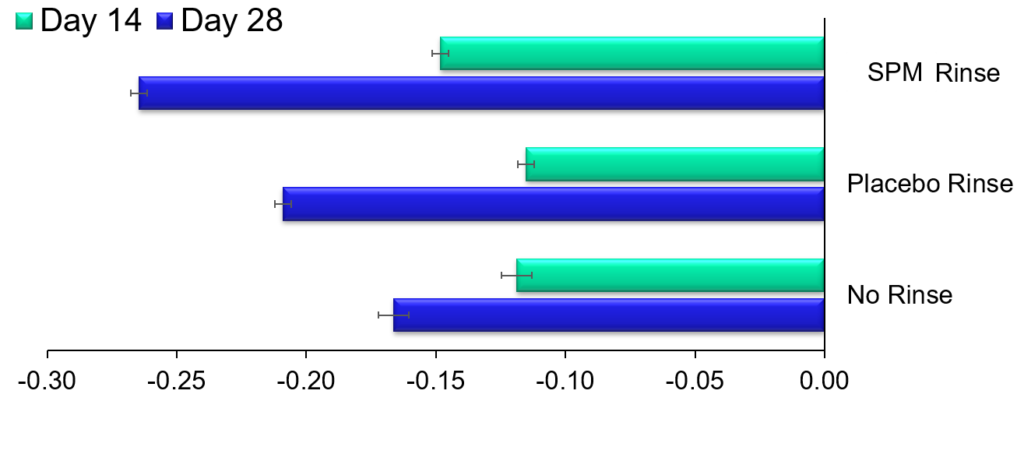
*Significant difference compared to placebo rinse
+Significant difference compared to placebo rinse and no-rinse
^Significant difference compared to no-rinse
Excellent Results
No toxicity
Secondary efficacy outcomes:
-
reduces gingivitis
-
reduces pocket depth
-
